Publication of the results of clinical trials is essential for Cochrane. They are the basis for high quality and relevant systematic reviews, and thus for evidence-based decisions about health care.
Cochrane Belgium, Test Aankoop and Kom op tegen Kanker joined forces with TranspariMED on a report on the transparency of clinical trials in Belgium. The report is based on clinical trial registration data in EudraCT, a registry specifically for drug trials.
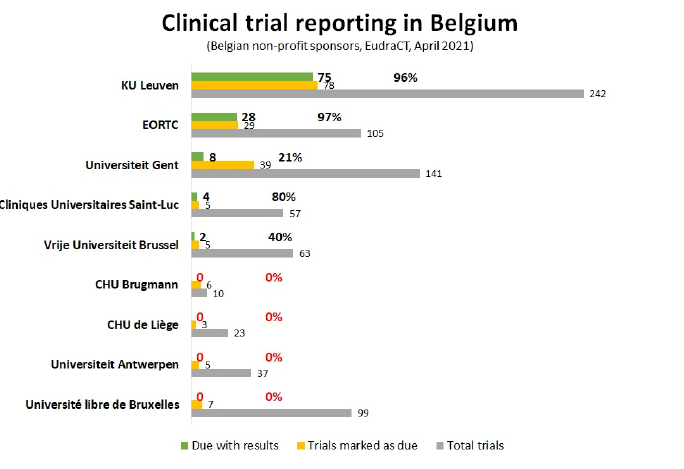
The report shows that 22% of verifiably due trial registrations have no results yet. However, this is probably an underestimation as only 292 of the 1098 registered Belgian trials have been marked as completed. The register contains many trials that started more than 10 years ago, which have not yet been marked as completed. It is unlikely that these studies are still ongoing.
Commercial organizations published their results much more often than non-commercial organizations such as universities and hospitals. The current report was published after reaching out to Belgian organizations last year asking them to register their results. Several organizations took this to heart and added results, but not all of them. There is certainly still room for improvement. Moreover, a more accurate picture needs to be formed of the trials that have been completed, this needs to be done by the national medicines regulator AFMPS together with the funding organizations. Results will be evaluated again in 6 months.