
In May 2021, Cochrane Belgium, Test Aankoop and Kom op tegen Kanker joined forces with Transparimed on a report of transparency of clinical trials in Belgium. As we announced then, we performed an update in December 2021.
Updated results showed that, overall, Belgian organizations made substantial progress. In the intermediate period, more than 50 studies have been added. Four sponsors, KU Leuven, European Organisation for Research and Treatment of Cancer (EORTC), Cliniques Universitaires Saint-Luc and CHU Brugmann have completed or almost completed the process of uploading all of their due clinical trial results. The other sponsors still have – to a greater or lesser extent – a long journey ahead. Out of 804 studies performed by the nine largest non-profit organizations, 218 are registered as being completed at least 1 year ago. These should all contain results.
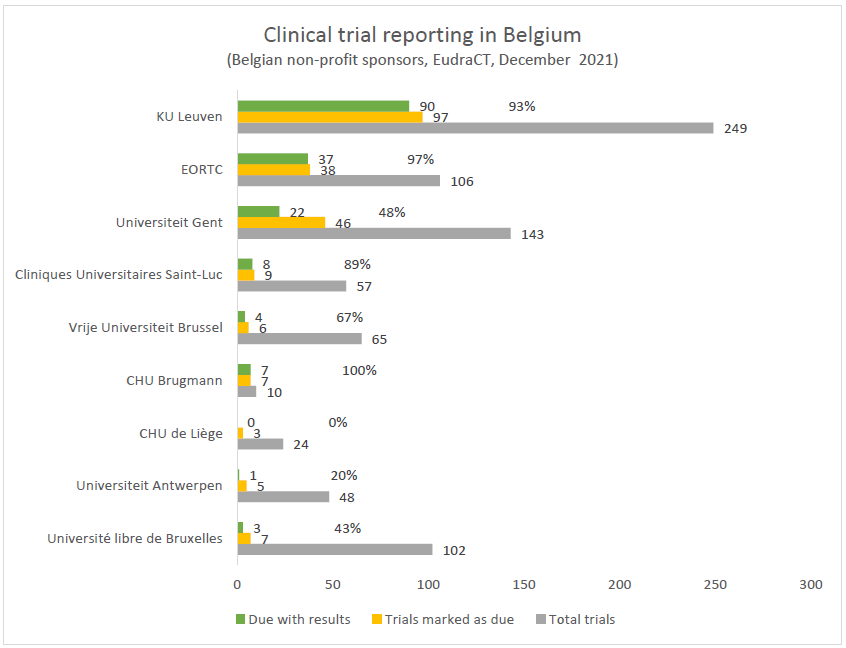
Results of 21% of these studies are still not uploaded in the register, and this number is an underestimation. This is an important problem, according to our co-director Trudy Bekkering, because you need access to all data to evaluate effects and possible harms of medication via systematic reviews.
Sanctions may be imposed for organizations that continue to fail registering their studies. However, we hope that this will not be necessary and that all organizations aim to get their remaining studies registered as soon as possible.
The full report is available here.
This news was picked up by Belgian newspapers De Morgen and Le Soir.
