What is the difference between a systematic review and a traditional review?
Reviews provide an overview of literature. In systematic reviews, the process from question to conclusion is transparent. Also the quality of all individual studies is assessed. This allows the reader to decide whether he or she has confidence in its conclusion. In traditional reviews, the method is typically not clear. For example, it is not clear whether all relevant studies were included or how the conclusion was formulated. Why do we even still publish traditional reviews?
Find out more about systematic reviews in this YouTube video.
What are Cochrane Systematic Reviews?
Cochrane reviews are literature overviews on a specific research question that are reliable and can be trusted. They are reliable because they are performed according to the highest standards in this field. Cochrane is a nonprofit organization that drives on volunteers all over the world. They aim to inform patients, clinicians and policy makers so they can make informed decisions. Informed decisions are based on scientific studies (and thus systematic reviews) instead of beliefs from a doctor, media or whoever.
Find out more about Cochrane evidence here.
What is the difference between a systematic review and a meta-analysis?
These terms are sometimes used as synonyms but this is not correct. A systematic review refers to a method of literature overview; i.e. systematic search and selection, assessment of the quality of the studies and a transparent presentation of results. Meta-analysis is a statistical method to combine the results of separate studies. It gives a sort of mean value across multiple studies. One can perform a systematic review and do a meta-analysis. Doing a meta-analysis without a systematic review will typically not make sense.
How do I know if a systematic review is of good quality?
A checklist exists with 16 items that reflect the quality of systematic reviews. It is called AMSTAR 2 (A Measurement Tool to Assess systematic Reviews 2). The items refer to how the systematic review was conducted, for example did the reviewers make a protocol beforehand, did they perform a comprehensive search, was the screening and selection done by two persons separately, etc. A systematic review is of good quality when all items are addressed. The AMSTAR 2 tool can also be used as a guide to performing a systematic review.
Should I perform a systematic review as part of my PhD thesis?
Yes, definitely!
More and more research proposals have to include a systematic review, according to funders. This is required for a good reason. Systematic reviews offer an overview of what is known, what the knowledge gaps are and identifies possible pitfalls in previous studies. Therefore, a systematic review is an excellent start for any PhD project.
Why do I have to write a protocol for my systematic review?
The protocol lays out the plans for your review. It is important to describe this beforehand in order not to make or change your plans based on the studies that are included in the review. This may compromise the quality of your review, because it pushes you in a certain direction. A good review keeps an open and unbiased view.
What is grey literature?
Grey literature covers all materials that are not published in books or journals. Examples are PhD theses, conference abstracts, working papers and technical reports. Because such materials are not published conventionally, they lack indexing in major electronic databases such as Medline. For this reason, finding grey literature for your systematic review is typically more difficult than finding published studies.
Which domains are suitable for systematic reviews?
All domains are suitable for systematic reviews. Systematic reviews are tools to answer a question, and this can be in any area, domain or field. For some domains, however, there is more expertise on the best methods to perform the review. This makes it easier to perform the review.
How to choose the scope of the review?
 The scope, or width, of a review may be narrow, broad or anything in between. An example of a broad review is “Exercise for patients with cardiac problems” This review would include all types of exercise and patients with a wide variety of cardiac problems. Consequently, the conclusion of such a review will be very general. Narrow reviews focus on specific interventions or populations, for example: “Dynamic leg exercises for elderly female smokers who experienced a heart attack?” Such reviews may include few, or sometimes no, studies. The conclusion of such a review will be very detailed; it applies only to that specific type of exercise for that specific group of patients. Mostly, the clinical relevance of a research question determines the optimal scope of a review.
The scope, or width, of a review may be narrow, broad or anything in between. An example of a broad review is “Exercise for patients with cardiac problems” This review would include all types of exercise and patients with a wide variety of cardiac problems. Consequently, the conclusion of such a review will be very general. Narrow reviews focus on specific interventions or populations, for example: “Dynamic leg exercises for elderly female smokers who experienced a heart attack?” Such reviews may include few, or sometimes no, studies. The conclusion of such a review will be very detailed; it applies only to that specific type of exercise for that specific group of patients. Mostly, the clinical relevance of a research question determines the optimal scope of a review.
Are empty reviews useful?
Empty reviews are systematic reviews that do not have any included studies. They may be useful as they illustrate that there are no (good-quality) studies available for a certain question. This may facilitate the conduct of new studies. However, such reviews are less useful if the review is empty because the inclusion criteria are very strict (and do not match clinical practice).
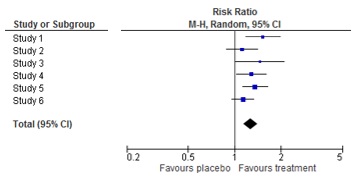
What is a forest plot?
A forest plot is a picture of a meta-analysis. It shows the results of each individual study as well as the overall result of the meta-analysis. For each study, the effect estimate and the confidence interval are shown. The combined result is at the bottom of the graph, shaped like a diamond. This represents the overall effect estimate and confidence interval. The name ‘forest plot’ originates from the forest of lines in the picture.
More information on the origin of forest plots can be found here.
What is the PRISMA checklist?
PRISMA is short for Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The PRISMA checklist contains 27 items with guidance on how to report on the item. PRISMA helps authors of systematic reviews and meta-analyses to report their work in a transparent way. More and more journal editors ask authors to adhere to this checklist when submitting a systematic review for publication. The PRISMA statement appeared in several publications, for example: http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000100
An update of the PRISMA statement is currently ongoing. A preprint of PRISMA 2020 is available here.
What is a (PRISMA) flowchart?
A (PRISMA) flowchart is a diagram that helps you present the results of the search of a systematic review in a standard way. It reports how many records were identified, how many, in the end, were included in the review and all stages in between. Such a flowchart is recommended as part of the reporting of any systematic review. See also the PRISMA statement (Q23).
What is evidence mapping?
Evidence mapping is one of the newest types of systematic reviews. Such reviews aim is to identify gaps in knowledge or future research needs. Characteristic to evidence mapping is a systematic search of a broad field. The results are typically presented in a user-friendly format, with or without a visual help such as a figure, graph, or a database. The methodology of such reviews is in its infancy.
What are living systematic reviews?
 Living systematic reviews are a new type of systematic reviews that are updated whenever new evidence is available. The aim is to improve the currentness of systematic reviews, which means to shorten the time between the publication of studies and their incorporation in systematic reviews. Obviously, the best methods for performing the review are still being used.
Living systematic reviews are a new type of systematic reviews that are updated whenever new evidence is available. The aim is to improve the currentness of systematic reviews, which means to shorten the time between the publication of studies and their incorporation in systematic reviews. Obviously, the best methods for performing the review are still being used.
Living systematic reviews are especially useful for topics where:
- there is important uncertainty concerning the evidence;
- new evidence is expected, illustrated by e.g. protocols of trials published in trial registers;
- the research question is important for policy or practice.
A Cochrane webinar is available here.
Do we really need to work in parallel to conduct a good systematic review, and during which steps is this most important?
Preparing and performing a systematic review takes many decisions. Doing parts of the review in duplicate reduces the risk of making mistakes. It also reduces the possibility that the beliefs of one reviewer affect the decisions, which may cause bias. Working in parallel increases the quality of your systematic review.
For Cochrane reviews, working in parallel is mandatory when making inclusion decisions for studies, when extracting outcome data and when assessing risk of bias. It is highly desirable during extracting study characteristics (MECIR standards: C39, C45, C46, C53).
What are MECIR standards?
MECIR is short for Methodological Expectations of Cochrane Intervention Reviews. These are methodological standards for Cochrane protocols, reviews and updates, which describe how you should conduct and report such reviews. These standards focus on systematic reviews of interventions.
The standards provide authors of Cochrane systematic reviews with clear and transparent guidance on all steps in the process. They are also useful for authors of non-Cochrane reviews as they describe how you should perform a high-quality systematic review.
New topics in Cochrane Handbook: equity
Health inequalities are differences in health within or between certain groups of people. These differences have a larger effect on those who are disadvantaged. Health inequality is avoidable and unfair. An example is socio-economic differences in asthma rates in children due to differential distribution of air pollutants. Increasing health equity is an important public policy objective.
Reviewers more and more incorporate equity in their review. Reviewers may, for example consider equity when they define their review question, or consider possible implications for their Summary of Findings table. The Equity Methods group provides useful information on how to incorporate equity in a systematic review. You can read the chapter on equity in the updated Cochrane Handbook. Is equity something to consider for your review?